Since the Valsartan recall on July 2018, nitrosamine impurities such as N-nitrosodimethylamnie (NDMA), N-nitrosodiethylamine (NDEA), N-nitroso-N-methyl-4-aminobutyric acid (NMBA), N-nitrosodibutylamine (NDBA) have been successively detected in sartan and non-sartan drugs. Nitrosamine impurity investigation is mandatory for studies of chemical drugs. On May 8, 2020, the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) issued the communication "Technical Guideline for Study of Nitrosamine Impurities in Chemical Drugs (for Trial Implementation)" (No. 1, 2020). The communication recommended to refer to the relevant provisions of ICH M7 (R1) guideline to ensure adequate and reasonable scientific basis of finally proposed control strategies and impurity limit; however, it did not specify a guideline for acceptance criterion establishment. This article discusses the establishment of acceptable limits for nitrosamine impurities.
1.Acceptable limits specified by FDA
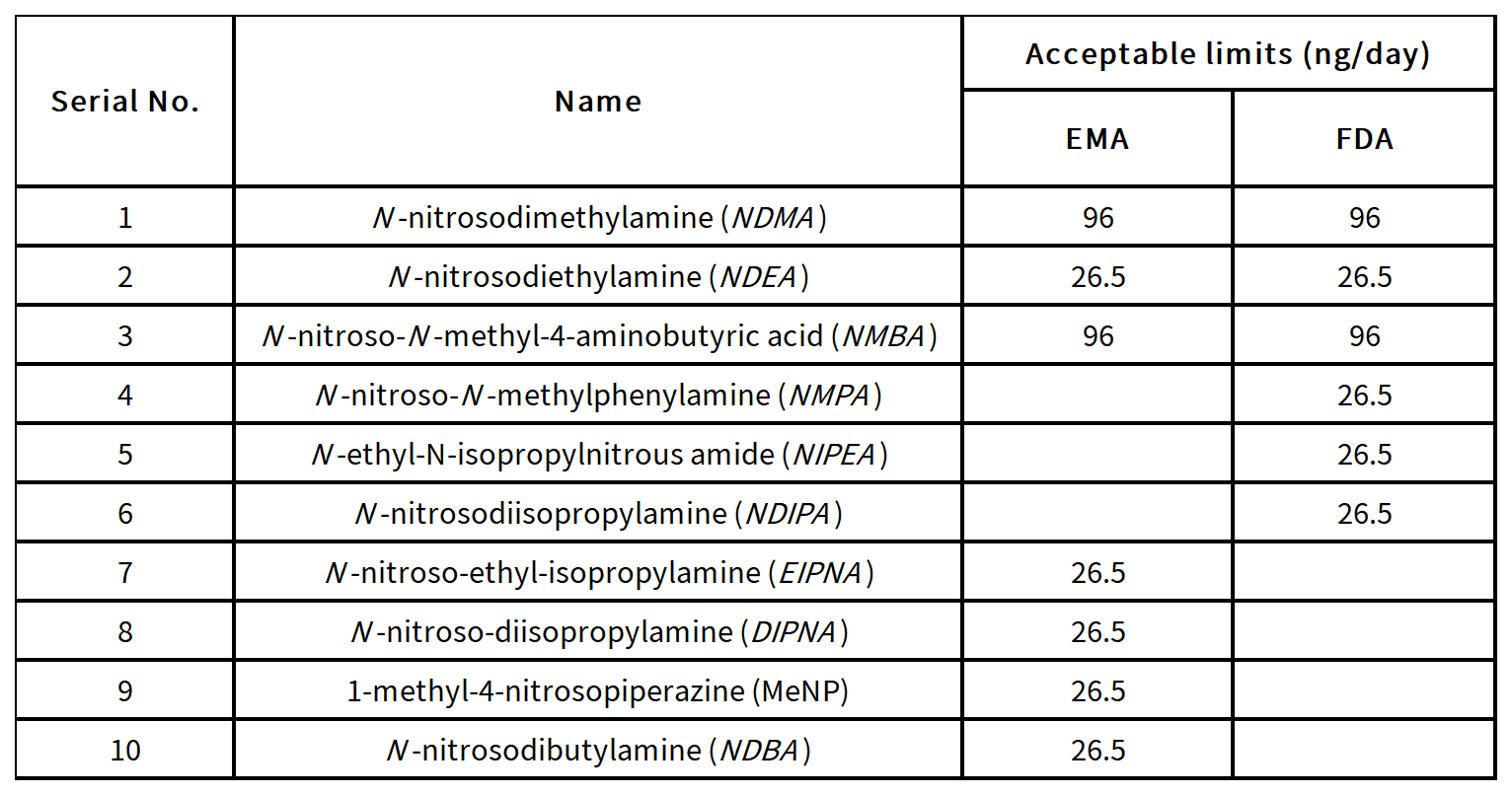
2.Predicted carcinogenic potency categorization approach
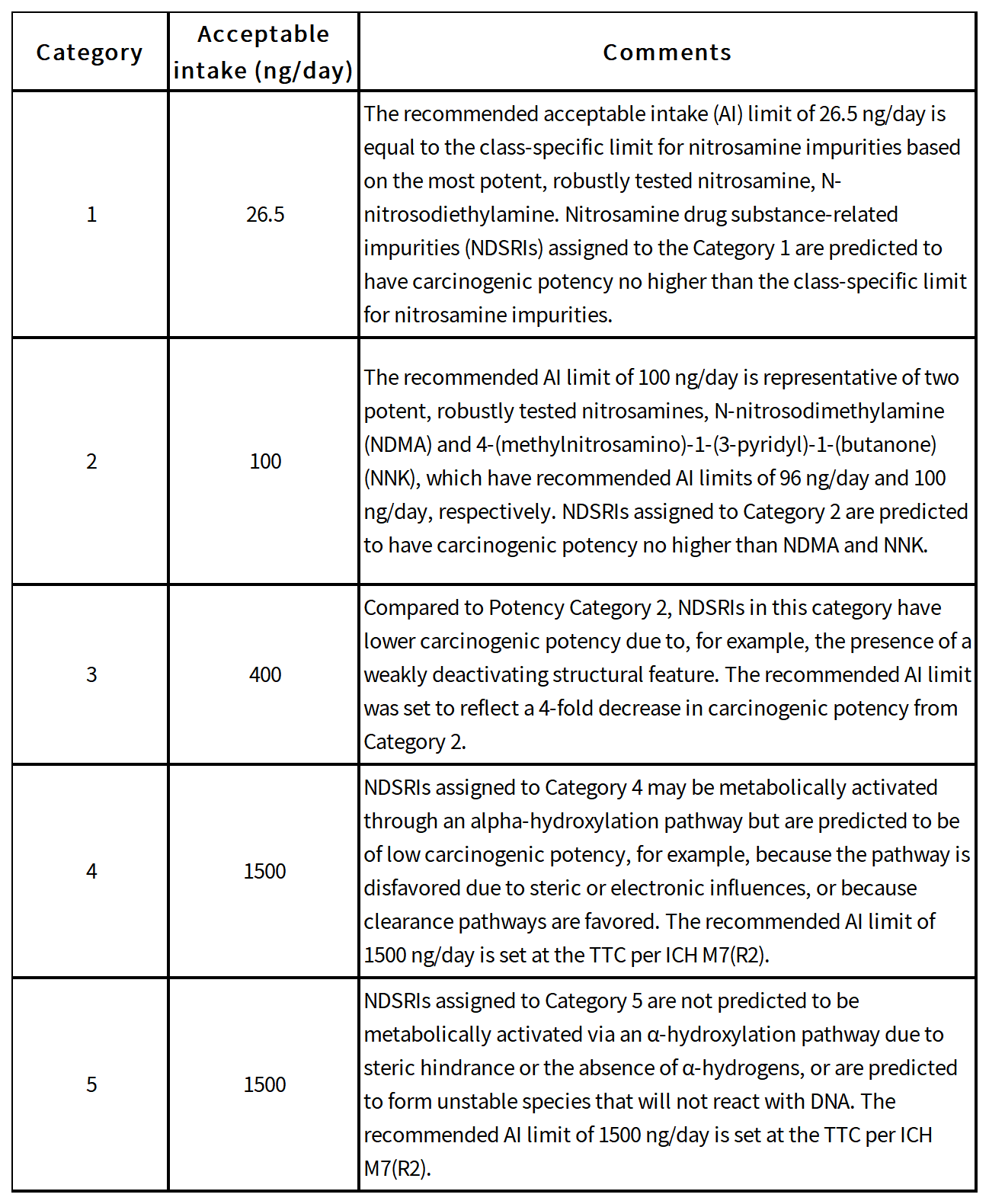
Nitrosamine impurities have been categorized into 5 categories by FDA according to predicted carcinogenic potency.
The decision tree for nitrosamine impurities are as follows:
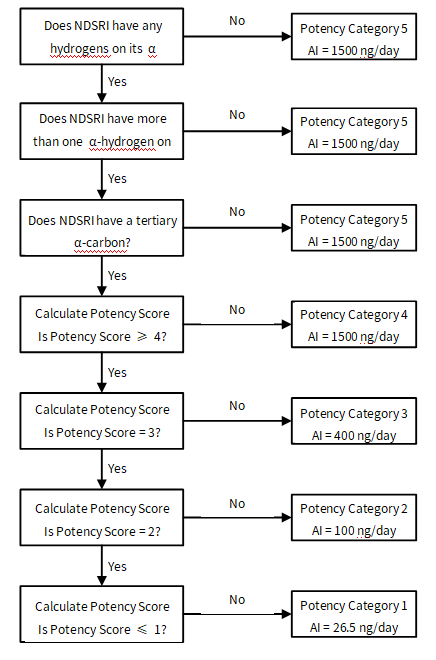
Determination of Potency Score
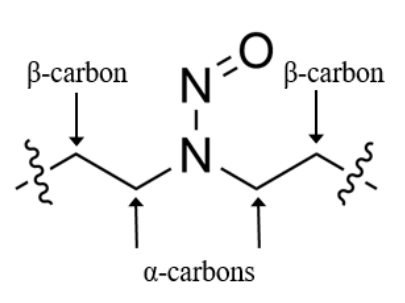
Potency Score = α-hydrogen score + deactivating feature score (sum all scores for features present in NDSRI) + activating feature score (sum all scores for features present in NDSRI)
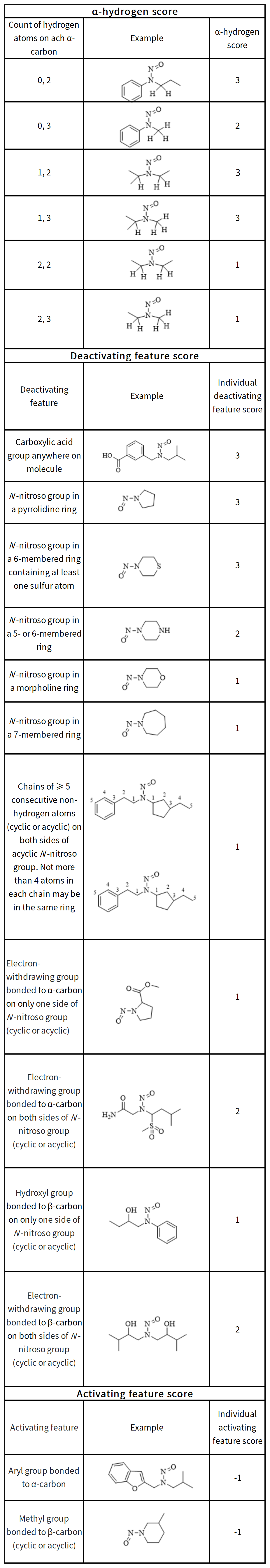
References
[1]Guidance for Industry.Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) [EB/OL]. (2023-08) [2023-08]. Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) (fda.gov)
[2]Guidance for Industry.Control of Nitrosamine Impurities in Human Drugs [EB/OL]. (2020-09)[2021-02]. download (fda.gov)
[3]Technical Guideline for Study of Nitrosamine Impurities in Chemical Drugs (for Trial Implementation) [EB/OL] (2020-05-08)[2020-05-08].Communication from the Center for Drug Evaluation of the National Medical Products Administration on issuance of "Technical Guideline for Study of Nitrosamine Impurities in Chemical Drugs (for Trial Implementation)" (No. 1, 2020) (cde.org.cn)
[4]Nitrosamine impurities in human medicinal products [EB/OL]. (2020-06-25)[2020-06-25]. Nitrosamines EMEA-H-A5(3)-1490 - Assessment Report (europa.eu)

