Genotoxic impurities (GTIs) refer to a class of impurities in drugs that can directly or indirectly cause DNA damage and gene mutations, with carcinogenicity or potential carcinogenicity. To ensure the quality and safety of drugs, the European Medicines Agency (EMA) introduced regulations on the management of GTIs in 2002. Since then, organizations such as the US Food and Drug Administration (FDA) and the International Conference on Harmonisation (ICH) have also issued a series of guidelines on the definition, classification, limits, testing, and risk assessment procedures of GTIs. At present, GTIs have become one of the key indicators for drug approval and marketing, and also one of the top concerns for R&D personnel. By analyzing and summarizing the identification, classification, hazard assessment methods, limits, and other aspects of GTIs, this paper provides references for the control of GTIs.
1.Assessment and classification of impurities
The impurities actually generated or potentially existing during the synthesis and storage of the new drug substance and during the production and storage of new formulations are assessed. Due to the frequent presence of special groups that may induce gene mutations or carcinogenesis, the structure of GTIs is referred to as alerting structure. Depending on their mutagenicity and carcinogenicity, GTIs are divided into five categories in the Assessment and control of DNA reactive(mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk (ICHM7): ① known mutagenic carcinogens; ② known mutagens with unknown carcinogenicity; ③ alerting structure, unrelated to the structure of the drug substance; no mutagenicity data; ④ alerting structure, same alert in drug substance or compounds related to the drug substance which have been tested and are non- mutagenic; ⑤ no structural alerts, or alerting structure with sufficient data to demonstrate lack of mutagenicity or carcinogenicity.
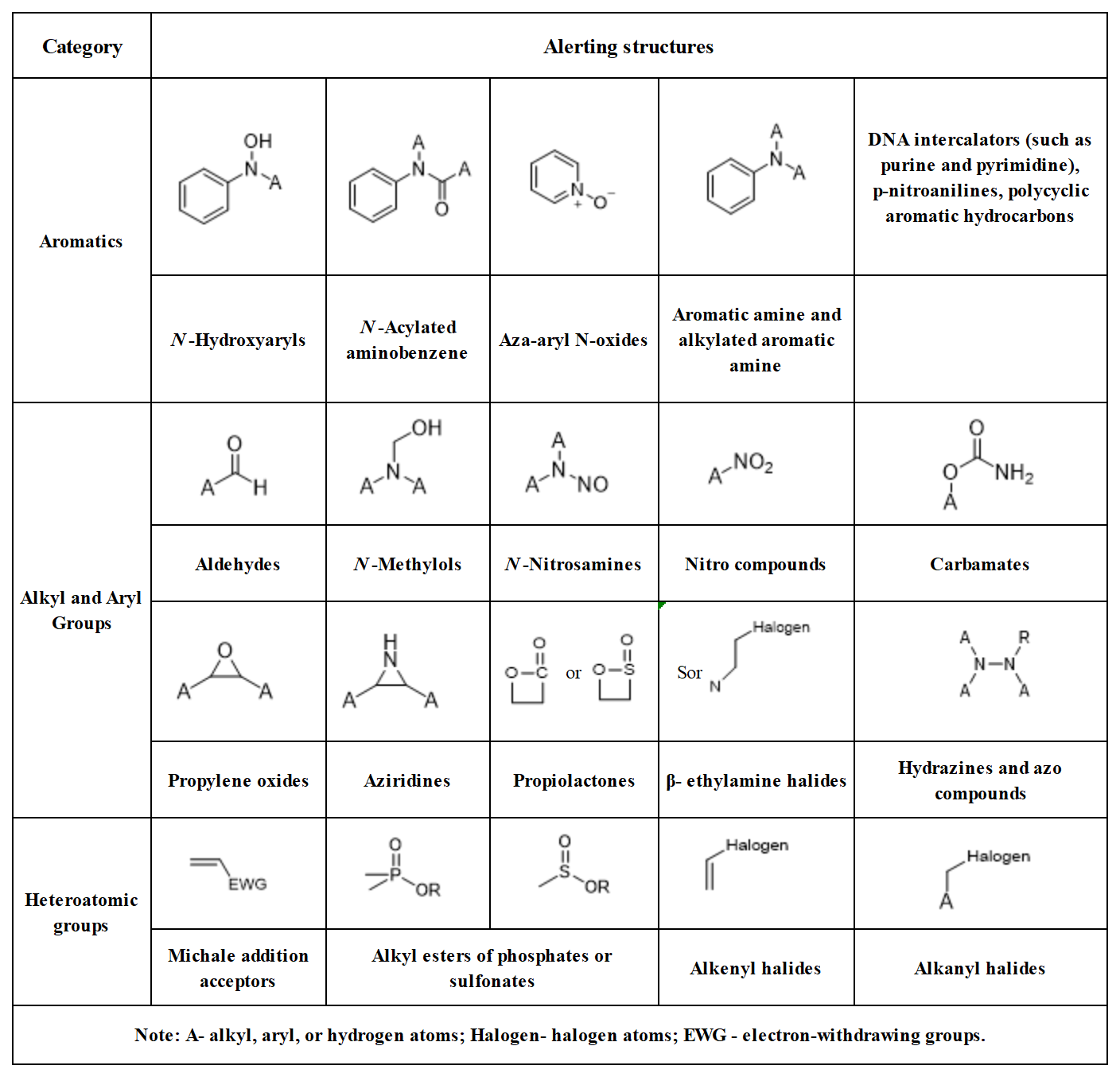
Table of common alerting structures
2.Classification of impurities
Before classifying an impurity, the first step is to determine whether it contains an alerting structure. If so, we can figure out whether it is carcinogenic by consulting the Toxicology Data Network (TOXNET)(National Library of Medicine - National Institutes of Health (nih.gov)), the Clinical Pathway Database (CPDB) (https://toxnet.nlm.nih.gov/cpdb/), the Chemical Toxicity Database (drugfuture.com)), Lhasa Carcinogenicity Database (lhasalimited.org), etc. For impurities not included in the databases of carcinogenic compounds, we can perform computational toxicology assessment using two (Quantitative) Structure-Activity Relationship ((Q) SAR) prediction methods that complement each other. One method should be expert rule-based and the second method should be statistical-based. For those who question the (Q) SAR positive method, further bacterial mutagenicity assay can be conducted. Please refer to the following map for details.
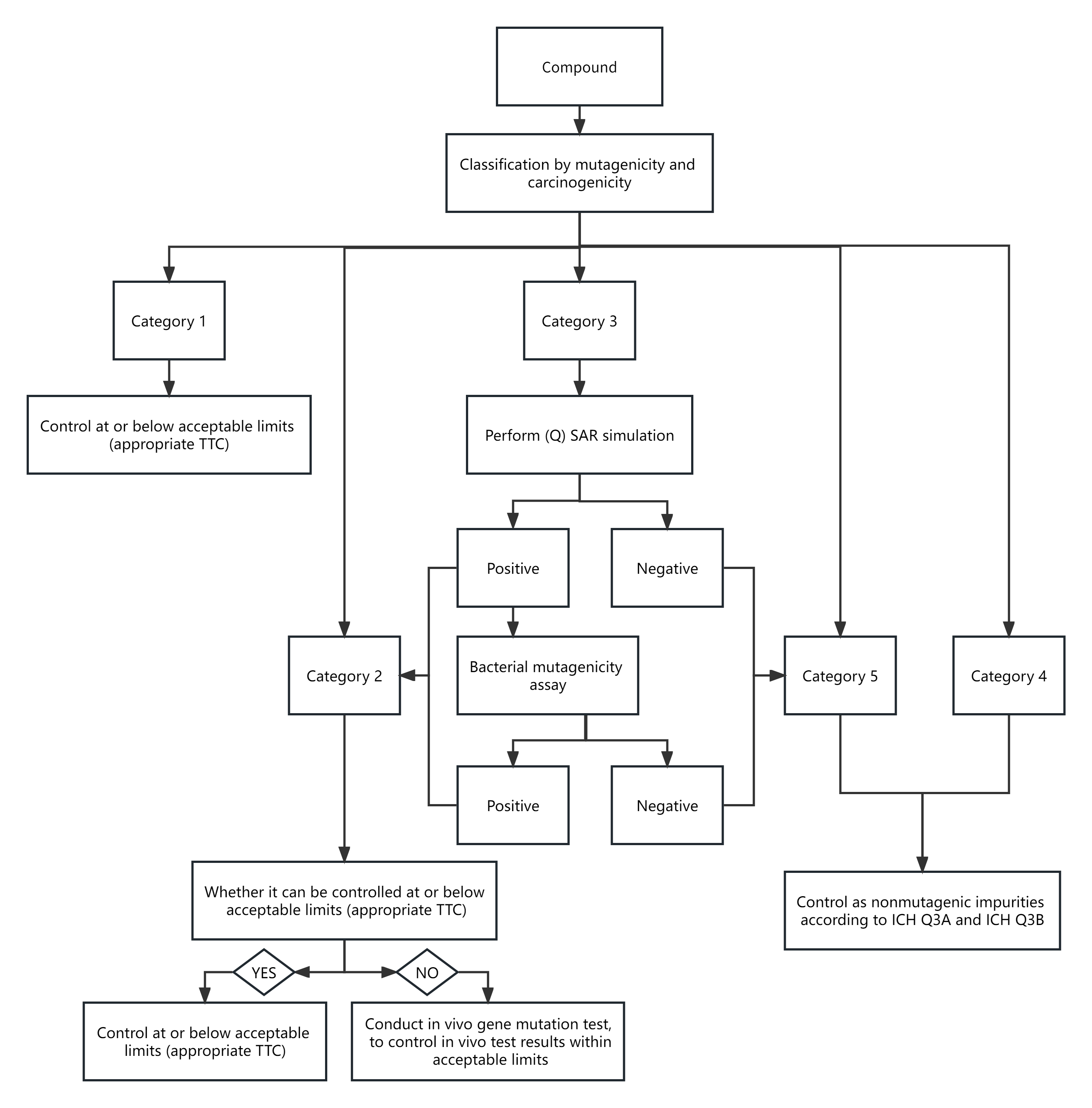
3.Establishment of acceptable limits for impurities
Category-1 impurities such as aflatoxin and hydrazine should comply with the specific acceptance criteria in ICH M7 or toxicity databases.
Category-2 impurities should be controlled within the acceptable limits (appropriate TTC). The TTC values for different dosing cycles are as follows:
Acceptable intakes of individual impurities:
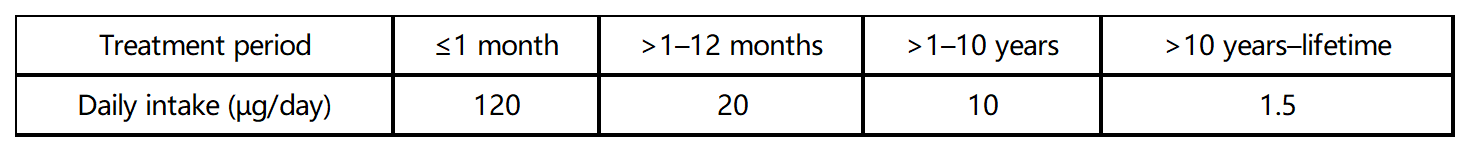
Total acceptable intake of multiple impurities:
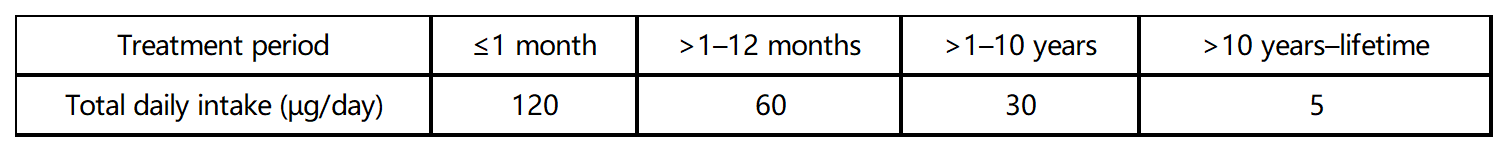
If the level of an impurity cannot be controlled within acceptable limits, in vivo genetic mutation test should be conducted to determine its accurate TTC and then establish its acceptance criteria based on the TTC.
As regards Category-3 impurities, the first step is to classify them using the above method, and then establish their acceptance criteria in accordance with the corresponding requirements.
The acceptance criteria of Category-4 and 5 impurities should be established in accordance with the requirements of ICH Q3A and ICH Q3B.
References
[1] ICH M7( R1).Assessment and Control of DNA Reactive ( Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk[EB/OL].(2017-05-31) [2020-02-03].https://database.ich.org/sites/default /files/M7_R1_Guideline.pdf
[2] Wan Junyue, Chen Hua, Yin Jie. Research progress on genotoxicity assessment strategies and related analysis methods for impurities in chemical drugs [J]. Chinese Journal of Pharmaceutical Analysis, 2022, 42 (4): 557–571.
[3] Sun Baihao, Li Wenlong, Guan Haoyue, et al. Research progress on genotoxic impurities in generic products [J]. Clinical Medication Journal, 2022, 20 (2): 8–12.

