Laviana Pharma (Cangzhou) Co., Ltd.
Founded in 2017, Laviana Pharma (Cangzhou) Co., Ltd. ( “Laviana (Cangzhou)”) is positioned as a benchmark company specialized in customized development and production of small molecule innovative drug APIs and advanced intermediates. We established the GMP production standards as required by FDA, EMA and NMPA in compliance with the ICH Guideline, which are aligned with all applicable laws, rules, regulations and regulatory documents related to environmental protection and workplace safety in the People’s Republic of China.
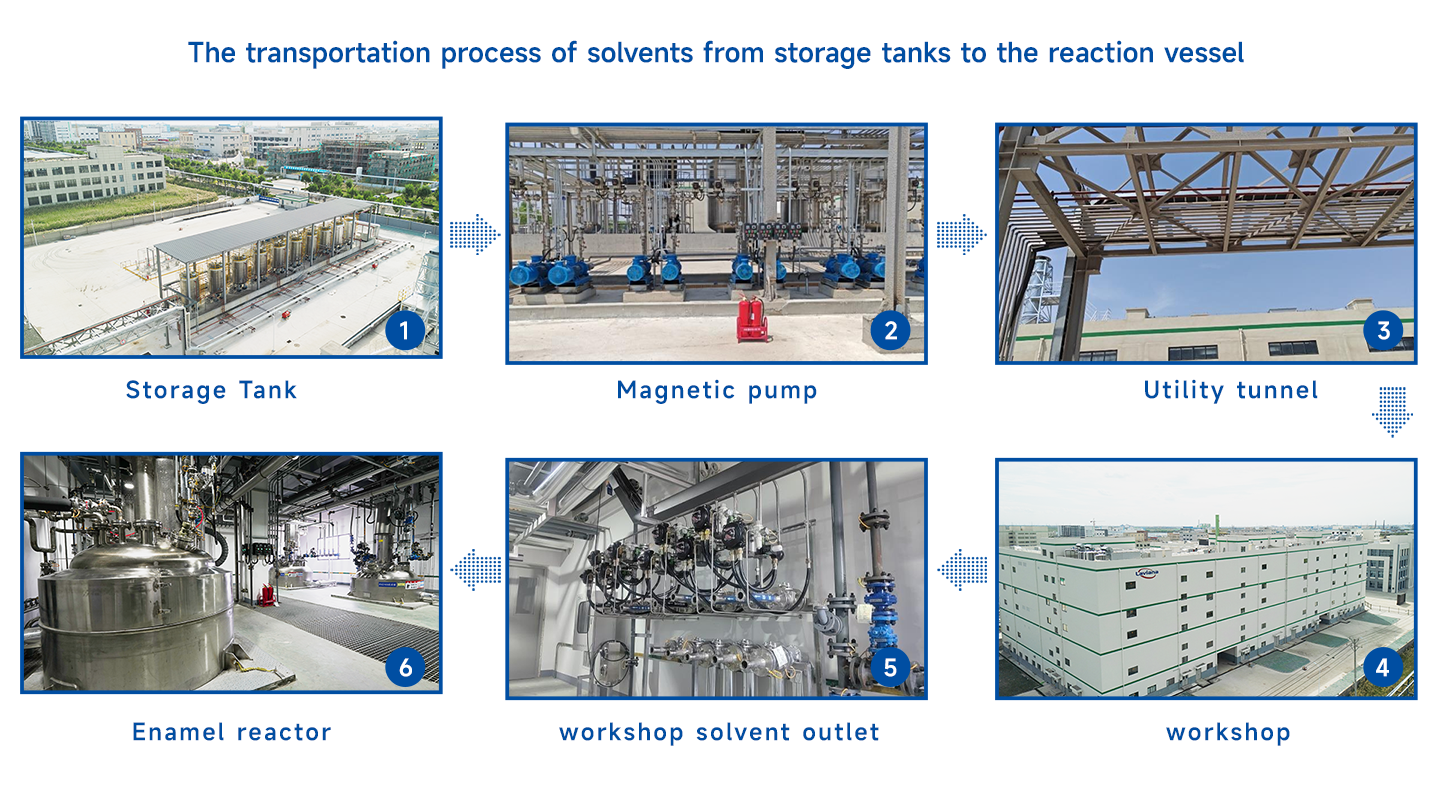
Situated at Lishizhen Road, West Area of Cangzhou Lingang Economic and Technological Development Zone, Laviana (Cangzhou) covers an area of 66,000.00 square meters with gross floor area of 48,793 square meters, of which the construction scale of Phase I is about 33,000 square meters. Our automated multi-functional workshop contain 6 pharmaceutical intermediate production lines, 3 general API production lines, 1 high potency API production line, and 1 hormone intermediate & API production line. Our Phase I production workshop have 65 reactors with a total volume of 106,700L that can function from -80℃ to 280℃. We’ve enhanced automatic, digital and intelligent level of pharmaceutical production by integrating data information across stages through the Manufacturing Execution System (MES), and automatic control in production processes by incorporating the DCS, SIS, GDS and FMCS for function, safety and process data recording.